

This explains why, if you heat sugar syrup, you get caramel at a given temperature almost instantly - you have reached the temperature where over 99% of your molecules will convert to the caramelized state after a second - but if you leave sugar for a very long time at lower temperatures, it will also caramelize. Per the central limit theorem, out of the millions of molecules in your food, the above distribution also tells you what percentage of them will be converted to the cooked state after a second.
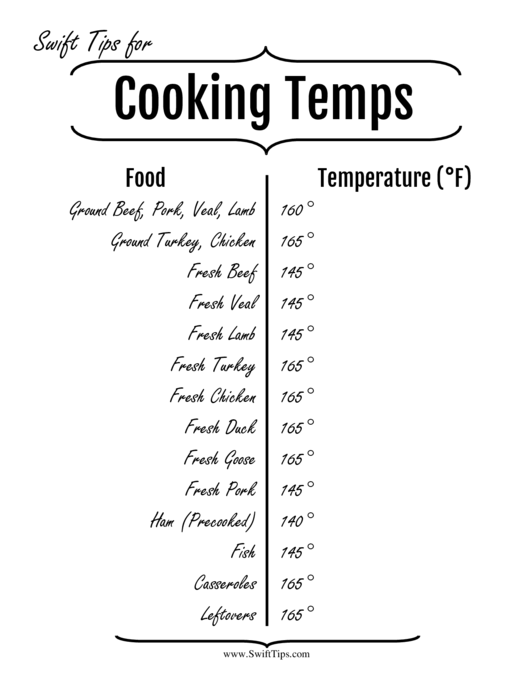
The probability of a molecule being denatured after a constant amount of time, say 1 second, should be roughly following a Gaussian distribution, depending on the temperature of the food (higher temperature -> the molecule shakes and moves more, and bumps more into other molecules, which makes the weak ternary and quarternary bonds snap): This means that you start with the rather curly protein molecule, and after it having suffered enough brownian movement, it unravels a little bit, losing some of the weaker bonds between atoms. Cooking food with heat is waiting for certain thermodynamic changes to happen, for example in the case of meat, you wait for the proteins to denature. Internal temperatureĬonsider first the easier part of the process: the relationship between the food's internal temperature and the doneness of the food. Even if you were to account for the fact that temperature is measured on a ratio, not interval scale where the real zero is at 0 Kelvin, it will still not help you at all. It is true that there is a negative correlation between cooking time and temperature: the higher the temperature, the shorter the cooking time.
#COOKING TEMPERATURE CONVERSION TRIAL#
So, cooking instructions are calibrated by trial and error (and educated intuition) to allow the different chemical and physical processes to happen in the conditions that produce best flavor and texture. While cooking in water you have a maximum temperature limit, at the boiling point. If you are cooking seeds like rice or beans, it takes a certain amount of time for the seeds to absorb water and become soft enough to eat, and this happens faster if the temperature is high. Somewhere in between you get the interior done properly, with the exterior just a little browned and crispy. You could turn it up to 500 and hope the inside heats up faster, but by the time the inside is ready, the meat on the outside gets way too hot and maybe even starts to blacken. If you want an internal temperature of 150 to kill bacteria or parasites, you could imagine cooking for 12 hours until the whole piece reaches that temp, but then you lose a lot of moisture. In general when dry-cooking meat you often want the inside to reach a certain temperature, without having the outside dry out too much. Then I can cook it for two minutes at a very high temperature to brown the surface without raising the overall temperature, so the inside stays rare. If I have a tough piece of meat, I might cook it for 12 hours at a low temperature and high moisture to tenderize it (and maybe in a braising liquid to add flavor). If gas production peaks before the temperature is high enough, the bubbles can collapse if the temperature rises too fast, the dough will set too early. The dough should set just as the bubbles are at their largest size for fluffy bread. It continues to produce gas as the heat begins to set the dough. These physical and chemical (even biological) processes require a certain optimal range of temperature (and humidity) and take a certain amount of time to be completed.įor example, when you bake bread, the yeast in the dough remains alive until the temperature rises high enough to kill it. Many "things" happen in cooking a particular dish.


 0 kommentar(er)
0 kommentar(er)
